- Resources
- …
- Resources


- Resources
- …
- Resources

AimGel Technology
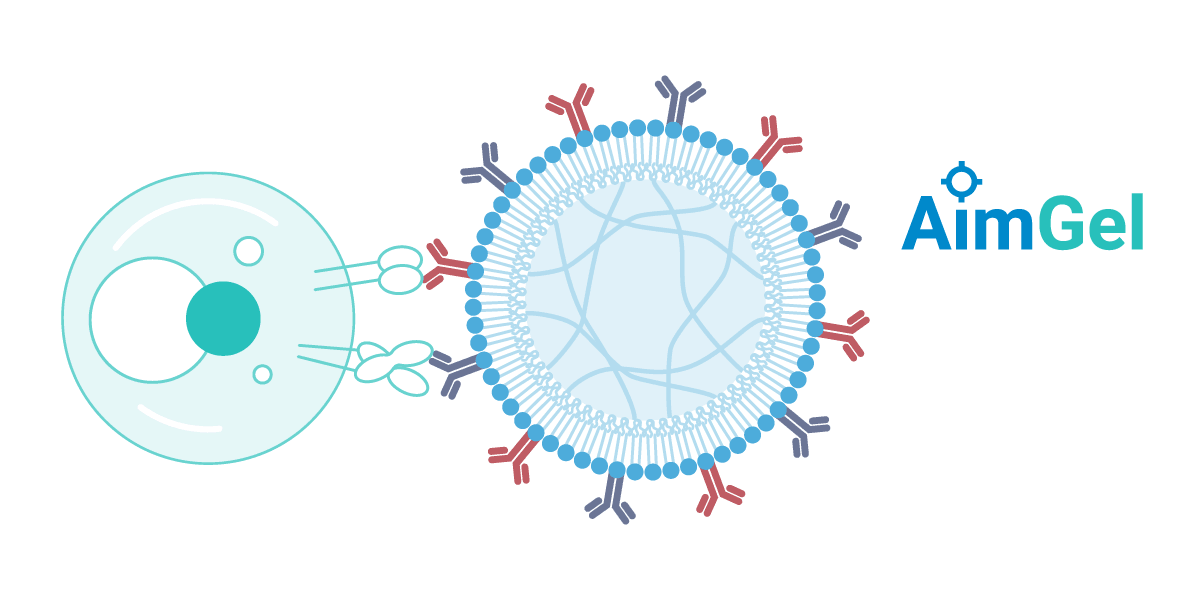
A modular artificial cell platform
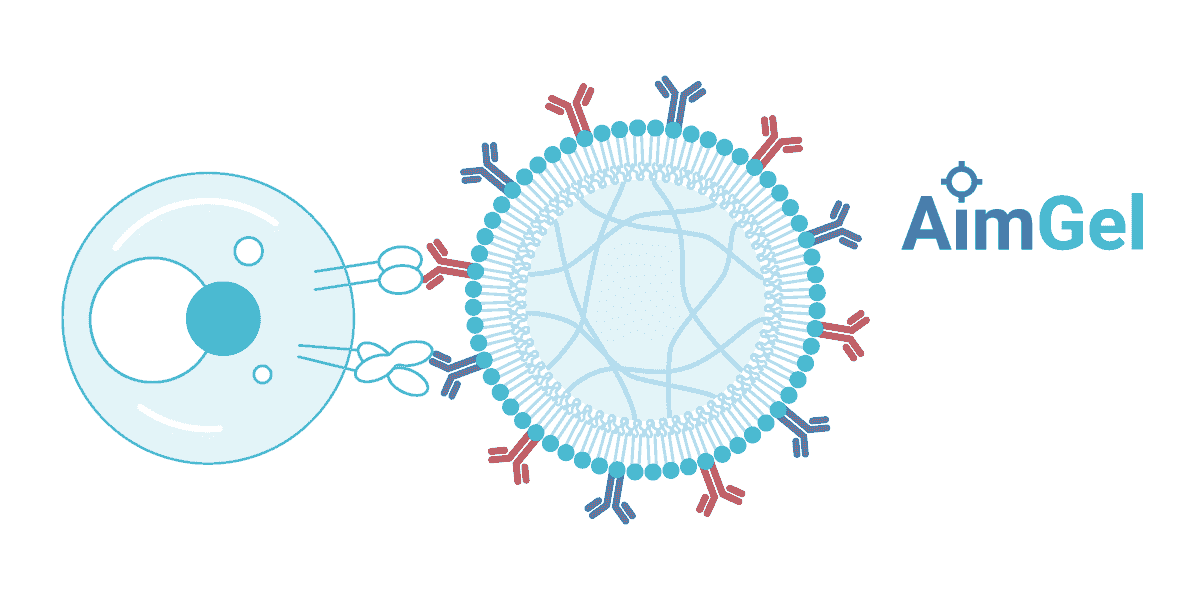
Biomimetic design
AimGel artificial cells are designed from the ground up to mimic natural antigen presenting cells (APCs). The soft hydrogel core replicates the mechanical stiffness and size (~10-15 µm) of real cells, while a mobile lipid bilayer coating allows activation signals to move freely across the surface — just as they would on a natural APC membrane.
This biomimetic design delivers measurable advantages: AimGel requires significantly fewer activation signals than rigid magnetic microplastic beads while achieving more effective yet gentler activation. The result is higher cell expansion, better viability, and reduced exhaustion — cells remain responsive and can be re-stimulated.
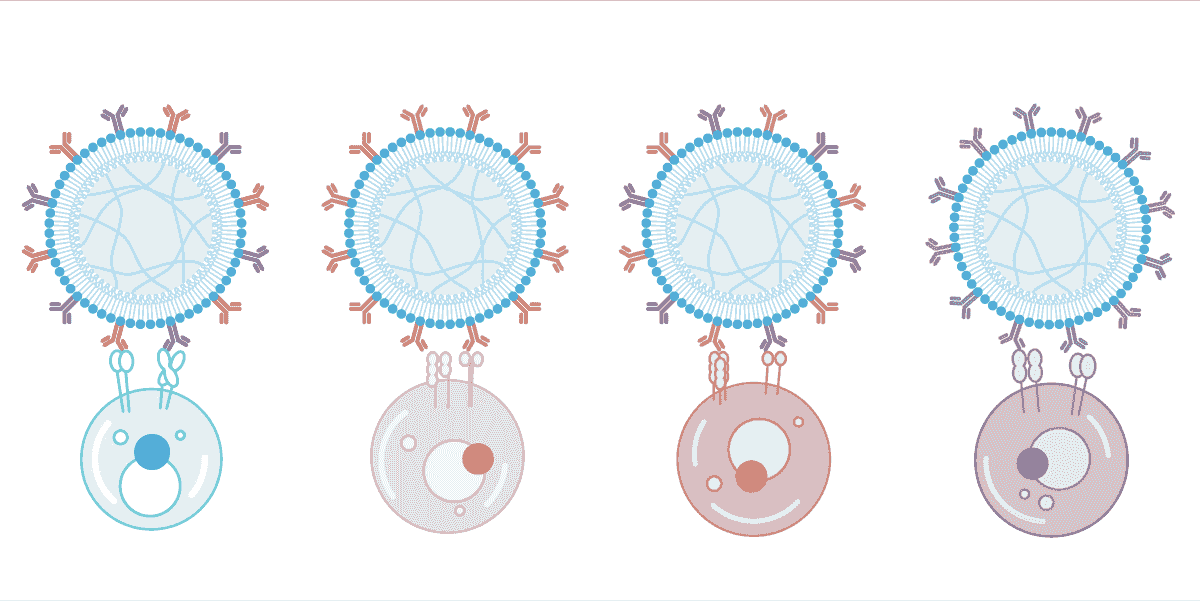
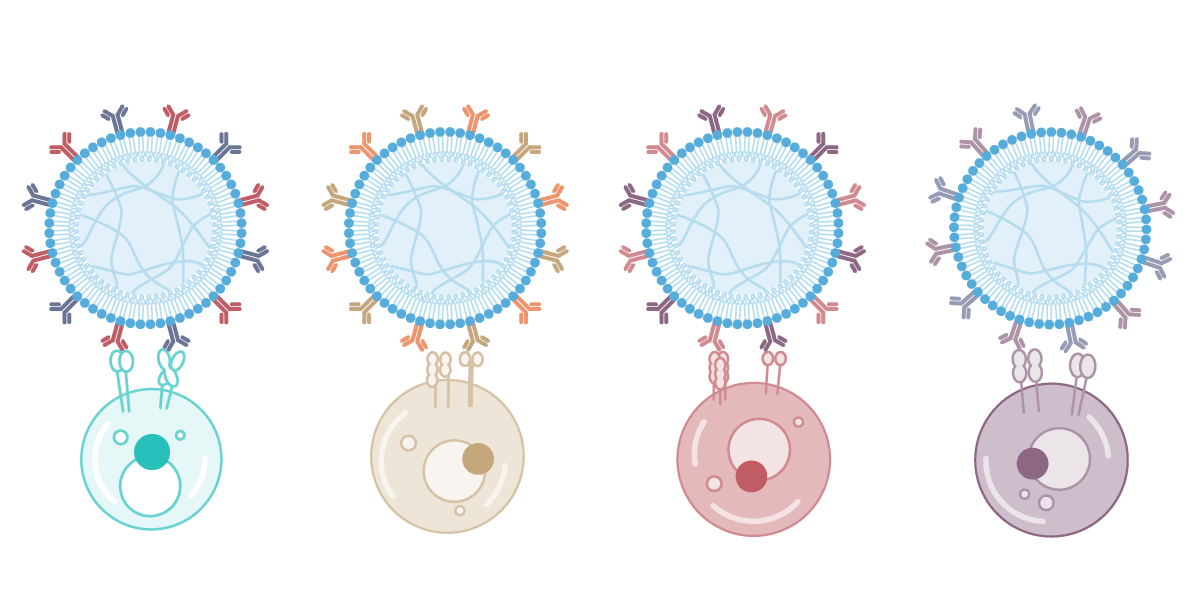
One platform, unlimited applications
AimGel's core advantage is its modular design. Degradation kinetics, bead size, and signal loading capacity are all chemically tuneable, giving you precise control over activation parameters. Load different signals for different experimental needs — currently optimised for T cell and NK cell activation, with a Treg solution coming soon.
The platform adapts to your research: adjust activation duration, coordinate with lentiviral transduction timing, or engineer entirely new signal combinations. One flexible platform, configured for your specific application.
Defined, consistent, compliant
AimGel is 100% synthetically derived with fully defined structures and ratios. No animal components means no batch-to-batch variability — just consistent, reproducible activation you can rely on for every experiment.

Fluid membrane
A fluid lipid coating mimicking the cell membrane enables cell-like interactions. Membrane is modified for signal attachment.

Hydrogel Core
A degradable hydrogel core with variable size and softness to mimic the texture of real cells.

Surface Signals
Easily swappable, optimised for your target cell type.
Journal Publications
- Chung JT & Chau Y. (2023). Self-adjuvanted L-arginine modified dextran-based nanogel for sustained local antigenic protein delivery to antigen presenting cells and enhanced cellular and humoral immune responses. (under review)
- Chung JT, Lau CML, Chung CH, Rafiei M, Yao S & Chau Y. (2023). Vaccine delivery by zwitterionic polysaccharide-based hydrogel microparticles showing enhanced immunogenicity and suppressed foreign body responses. Biomaterials Science, 11(14), 4827-4844.
- Jahanmir G, Lau CML, Yu Y & Chau Y. (2022). Stochastic Lattice-Based Modeling of Macromolecule Release from Degradable Hydrogel. ACS Biomaterials Science & Engineering, 8(10), 4402-4412.
- Chung JT, Lau CML & Chau Y. (2021). The effect of polysaccharide-based hydrogel on the response of antigen presenting cell line to immunomodulators. Biomaterials Science 9.19 (2021): 6542-6554.
- Chung CHY, Lau CML, Sin DT, Chung, JT, Zhang Y, Chau Y & Yao S. (2021). Droplet-Based Microfluidic Synthesis of Hydrogel Microparticles via Click Chemistry-Based Cross-Linking for the Controlled Release of Proteins. ACS Applied Bio Materials, 4(8), 6186-6194.
- Lau CML, Jahanmir G, Yu Y & Chau Y. (2021). Controllable multi-phase protein release from in-situ hydrolyzable hydrogel. Journal of Controlled Release, 335, 75-85.
- Jahanmir G, Lau CML, Abdekhodaie MJ & Chau, Y. (2020). Dual-Diffusivity Stochastic Model for Macromolecule Release from a Hydrogel. ACS Applied Bio Materials, 3(7), 4208-4219.